The Food and Drug Administration (FDA) has established guidelines for electronic records and electronic signatures in the Code of Federal Regulations Title 21: Food and Drugs PART 11 – Electronic Records; Electronic Signatures (21 CFR Part 11).
The regulation aims to facilitate extensive utilization of technology while upholding the integrity and security of electronic records and signatures, thereby reinforcing the FDA’s commitment to safeguarding public health.
In this blog post, we’ll cover the fundamentals of FDA Part 11. SIGN Butler is trusted by healthcare organizations seeking compliance with the stringent guidelines of the Code of Federal Regulations, Title 21, Part 11. It facilitates secure and compliant document generation processes, meeting the regulatory demands of the industry.
Who is required to be compliant with 21 CFR Part 11?
Organizations subject to 21 CFR Part 11 are those regulated by the FDA or involved in activities related to FDA-regulated products. Industries falling under this category include:
- Pharmaceutical companies
- Biotechnology firms
- Medical device manufacturers
- Contract research organizations (CROs)
- Contract manufacturing organizations (CMOs)
- Clinical laboratories
- Food and beverage manufacturers
- Cosmetics manufacturers.
Understanding FDA Part 11 Requirements:
When implementing a digital signature solution in accordance with FDA Part 11 regulations, Section 11.50 emphasizes the importance of Signature Manifestations.
This entails that all signed electronic records must include specific information associated with the signing, such as the printed name of the signer, the date and time when the signature was executed, and the meaning attributed to the signature (e.g., review, approval, responsibility, or authorship).
A unique identifier for each signer is also required. This adherence to legal requirements enhances the integrity and reliability of the digital signature process.
What are the requirements for electronic signatures?
The FDA embraces the use of electronic signatures as a modern alternative to traditional pen-and-ink signatures on paper documents, facilitating digital transactions.
To ensure compliance, electronic signatures must encompass the following elements:
- The printed name of the signer
- The date and time the signature was executed
- A unique user ID
- Digitally adopted signature
- Signing reason (meaning of the signature)

To determine if your digital signature solution complies with FDA Part 11 regulations on electronic records and electronic signatures, you’ll need to conduct a thorough assessment. FDA Part 11 sets criteria for using electronic records and signatures in place of traditional paper records.
Compliance generally involves several aspects, including:
- Validation: Ensuring that the system is validated for its intended use and that it maintains data integrity, accuracy, and reliability.
- Security Controls: Implementing access controls, audit trails, and measures to prevent unauthorized access or changes to data.
- Audit Trails: Creating comprehensive audit trails that capture all relevant data changes, including who made the changes and when.
- Digital Signatures: Establishing procedures to ensure digital signatures are unique, verifiable, and linked to the individual
- Record Retention: Ensuring that electronic records are secure, can’t be altered, and are available for review when needed.
SIGN Butler addresses these critical components of FDA Part 11 compliance, offering a robust platform tailored to meet the specific requirements of regulated industries.
From validation and security controls to electronic signatures and record retention, SIGN Butler streamlines the electronic signature process while ensuring adherence to regulatory standards.
Also, unlike other platforms, SIGN Butler allows you to tailor your signing page to further amplify your brand identity and enhance your customer experience.
Key Components of SIGN Butler:
Your data is yours to own and protect.
SIGN Butler is the first Salesforce digital signature tool that only stores your data in your own Salesforce.com organization.
Simple Configuration.
SIGN Butler simplifies configuration with its user-friendly interface, allowing users to effortlessly customize signature workflows, templates, and user permissions to suit their specific needs.
Easily track agreements.
Stay on top of your agreements with effortless progress tracking. Be informed about agreement stages andensure timely completion with automated reminders.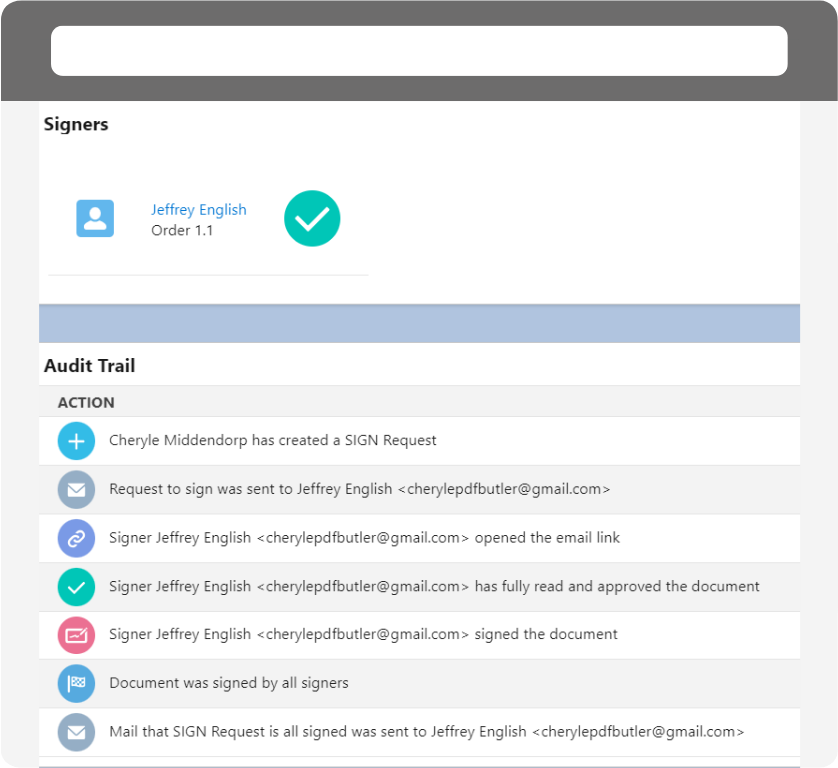
Audit Trail and Certificates
Ensure compliance with comprehensive audit trails and certificates of completion.

In conclusion, SIGN Butler emerges as a reliable solution for organizations seeking to achieve compliance with FDA Part 11 regulations on electronic records and signatures.
By addressing key components such as validation, security controls, electronic signatures, and record retention, SIGN Butler empowers organizations to streamline their electronic signature processes while upholding data integrity, security, and regulatory compliance.




